
Medical devices manufacturer, Sky Medical Technology, parent company of Firstkind announced the Brihanmumbai Municipal Corporation (BMC) Hospital has integrated the geko device into its physiotherapy regimes for neuro and orthopaedic rehabilitation.
Across the BMC hospitals group, the geko device combines use with existing treatments to improve spasticity and range of motion following stroke, and in lower limb pre- and post-operative oedema reduction following orthopaedic surgery and orthopaedic trauma. Alongside these applications, the device is also delivering the added benefit of deep vein thrombosis (DVT) prevention in at-risk immobile and medically ill patients.
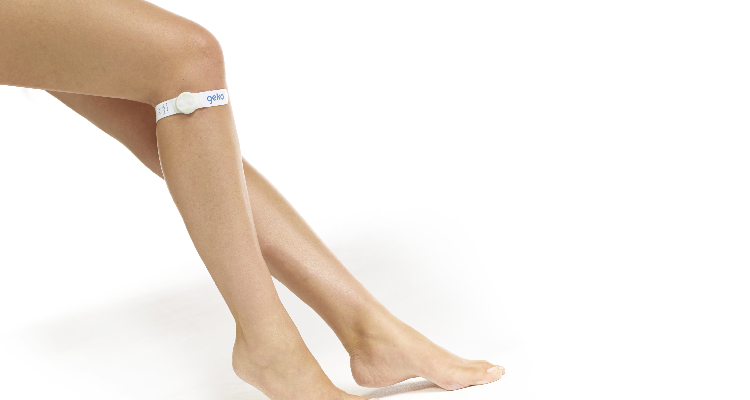
The geko device is a small, self-adhesive, wearable neuromuscular electro-stimulator that is applied to the surface of the skin just below the knee, over the head of the fibula bone. It delivers a gentle electrical pulse, once per second, to the common peroneal nerve, activating the calf and foot muscle pumps, increasing venous, arterial, and microcirculatory blood flow at a rate equal to 60 per cent (1) of walking, effectively replicating the effects of exercise.
Commenting on behalf of the BMC, Dr Haridas Rathod, Chief Medical Officer, stated, “At the heart of physiotherapy is the goal to enhance a patient’s functional abilities, promote self-reliance, and improve overall quality of life. The geko device delivers on all three. When used immediately following a stroke or orthopaedic surgery, adjunctive use of the geko device not only allows physiotherapy to begin sooner but enables continuation in the home setting. Its ease of use empowers patients to take control and self-manage their rehabilitation. By reducing the hands-on physiotherapist time, the geko device generates significant cost savings for the BMC.”
Commenting on behalf of Curamatix Healthcare, the distribution partner for the geko device in India, Ramen Mukherjee and Subhodeep Mukherjee, Directors, “The successful integration of the device into BMC Hospital’s physiotherapy programmes is a significant milestone that highlights the device’s versatility and effectiveness in supporting stroke and orthopaedic rehabilitation.”
Commenting on behalf of Sky Medical Technology, Bernard Ross, CEO and Founder, “By seamlessly complementing existing treatments, the geko device not only accelerates recovery but also empowers patients to take control of their rehabilitation journey.”
References:
- https://pubmed.ncbi.nlm.nih.gov/22477572/





